THC9
The effects of Δ9-tetrahydrocannabinol on the dopamine system
Acute THC causes increased dopamine release and neuron activity, whilst long-term use is associated with blunting of the dopamine system. Future research must examine the long-term and developmental dopaminergic effects of the drug.
Preface
Δ9-tetrahydrocannabinol (THC), the main psychoactive ingredient in cannabis, is a pressing concern to global mental health. Patterns of use are changing drastically due to legalisation, availability of synthetic analogues (‘spice’), cannavaping and aggrandizements in the purported therapeutic effects of cannabis. Many of THC’s reinforcing effects are mediated by the dopamine system. Due to complex cannabinoid-dopamine interactions there is conflicting evidence from human and animal research fields. Acute THC causes increased dopamine release and neuron activity, whilst long-term use is associated with blunting of the dopamine system. Future research must examine the long-term and developmental dopaminergic effects of the drug.
Introduction
Cannabis is a widely used recreational drug. Over half of young Americans have used the drug1. In Europe cannabis has now overtaken heroin as the most widely reported illegal drug used amongst people entering specialist addiction services2. At the same time, political debates about changes to the legal status of the drug continue internationally. Although causality has not been conclusively demonstrated, heavy cannabis use is associated with increased risk of mental disorder3 including psychosis4, addiction5, depression6, suicidality7, cognitive impairment8 and amotivation9.
Δ9-Tetrahydrocannabinol (THC), cannabis’ main psychoactive component10, elicits its acute psychoactive effects via the endocannabinoid type 1 (CB1) receptor (CB1R)11. THC has been linked to the rewarding aspects of cannabis and the induction of symptoms of mental illnesses and cognitive impairment. Lately the THC content of cannabis has been increasing12, synthetic THC analogues (potent cannabinoid agonists; termed ‘spice’) are now widely used13. The future consumption of cannabinoids through electronic cigarettes (‘cannavaping’) and edible products14 changes the landscape further15. Given the widespread use of cannabinoids, and the links between THC exposure and adverse outcomes, it is imperative to understand the neurobiological effects of THC. Recently, we and others have found that heavy cannabis use is associated with reductions in dopaminergic function. Since the rewarding and psychotogenic effects of THC and its analogues are thought to be mediated by the dopaminergic system, demonstrating dopaminergic alterations in vivo in human users is of clinical relevance for the prevention and treatment of cannabis use disorders and psychoses. Therefore, we review the animal and human literature on the complex effects of acute and longer-term THC on dopamine synthesis, release, and its receptors, critically analysing the factors that contribute to effects, and variations between studies, before finally providing a framework for future research including pharmacologically dissecting these effects, especially in the developing brain.
THC receptor binding in the brain
THC is a CB1R and endocannabinoid type 2 receptor (CB2R) partial agonist11. The psychoactive effects of THC are blocked by the CB1R antagonist rimonabant16,17 indicating that these are mediated through activating G-protein-coupled CB1R receptors which reduce cyclic adenosine monophosphate (cAMP) levels by inhibiting adenylate cyclase18. THC disrupts finely-tuned endocannabinoid retrograde signalling systems due to the temporal and neuronal specificity of endocannabinoids over THC. Under conditions of low CB1R density, THC antagonises endogenous agonists possessing greater receptor efficacy than THC19. THC also allosterically modulates opioid receptors20, which may provide additional indirect routes for altering dopamine transmission21. Furthermore, THC has psychoactive metabolites with CB1R affinity, further complicating the analyses of receptor binding studies22.
CB1 receptors and dopamine
Early animal studies described the interactions of amphetamine, which increases dopamine release, and THC23. These reported that amphetamine’s behavioural effects were potentiated or antagonised depending on the dose of THC leading researchers24 to propose that dopamine was “a prime candidate for…the mode of action of Δ9-tetrahydrocannabinol”. Indeed, THC produces complex effects on the dopamine system, contributing to the drug’s recreational and harmful effects. However, there are inconsistencies between the preclinical and clinical findings which challenge the field. It is thus timely to review the evidence and provide a framework for understanding the inconsistencies between the preclinical and clinical findings.
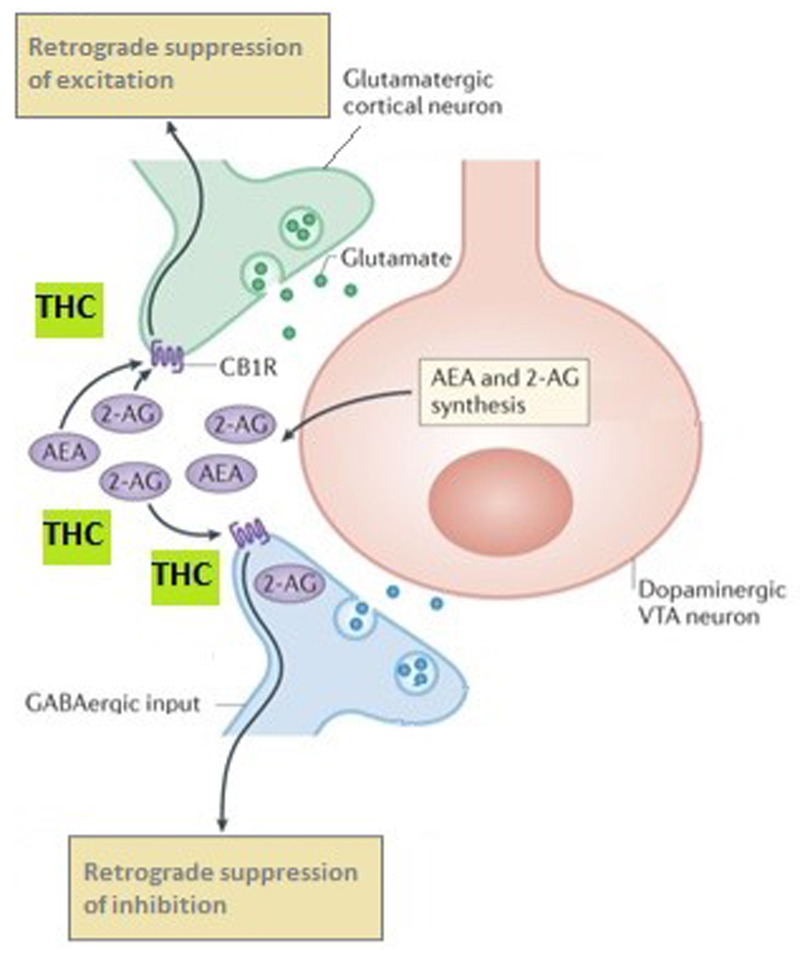
Dopaminergic neurons are modulated by the endocannabinoid system (eCBS)25. CB1Rs and the endocannabinoid ligands anandamide and 2-arachidonoylglycerol (2-AG) are abundant in dopaminergic pathways including the striatum26 where they act as a retrograde feedback system on presynaptic glutamatergic and γ-aminobutyric acid (GABA) nerve terminals (Fig. 1) to modulate dopamine transmission. Anandamide27 and 2-AG28 stimulate dopamine release in the nucleus accumbens (NAc) shell. This effect is blocked by the CB1 antagonist rimonabant, indicating that dopaminergic effects of endocannabinoids involve CB1 receptors. The rewarding properties of THC via increased dopamine release and dopaminergic neuron firing are underpinned by biased signal transduction mechanisms from the CB1R16. There is evidence of differential effects of acute vs. chronic THC exposure on the dopaminergic system. Therefore, we will treat these separately and describe the effects on different neurobiological components of the dopaminergic system including neuron firing, synthesis, release, reuptake and receptors.
Fig. 1. THC binds to CB1 receptors on glutamatergic and GABAergic neurons disrupting normal endocannabinoid retrograde signalling from dopaminergic neurons137.
Endocannabinoids (eCBs) influence ventral tegmental area (VTA) synaptic signalling. 2-Arachidonoylglycerol (2-AG) is synthesised by diacylglycerol lipase (DAGL) in dopaminergic VTA neurons and, once released, retroactively acts on endocannabinoid type 1 receptors (CB1Rs) on nearby glutamatergic and γ-aminobutyric acid (GABA)-ergic terminals. CB1Rs mediate robust inhibition of GABA inputs onto VTA dopamine cells29, termed retrograde suppression of inhibition. CB1Rs are also localized on glutamatergic terminals synapsing on VTA dopamine neurons30 where eCBs mediate retrograde suppression of excitation. Thus, eCBs fine-tune the activity of the mesolimbic dopamine projections through modulating both excitatory and inhibitory signalling. THC disrupts this finely tuned system.
Acute THC and presynaptic dopamine in animals
From the outset it was clear that THC exerts complex effects on the dopamine system. Early in vitro studies in rodents using radiolabelled dopamine in synaptosomes found that THC caused increased dopamine synthesis31 and release24 (Fig. 2). However, the effects on dopamine uptake yielded conflicting results, with evidence of both increases32 and dose-dependent decreases24. Subsequently biphasic and triphasic effects of THC were discovered, whereby low doses of THC produced increases in the conversion of tyrosine to dopamine, but high doses of THC resulted in decreased dopamine synthesis33. Likewise, complicated temporal relationships between THC administration and changes in dopamine levels were observed34, such that repeated dosing results in behavioural and neurochemical tolerance –highly pertinent to the mechanisms of dependence to the drug. The complex dose-specific effects of THC in rodents were thought to be due to dose-related decreases in precursor uptake32 and dopamine-opioid interactions via μ-opioid receptors31.
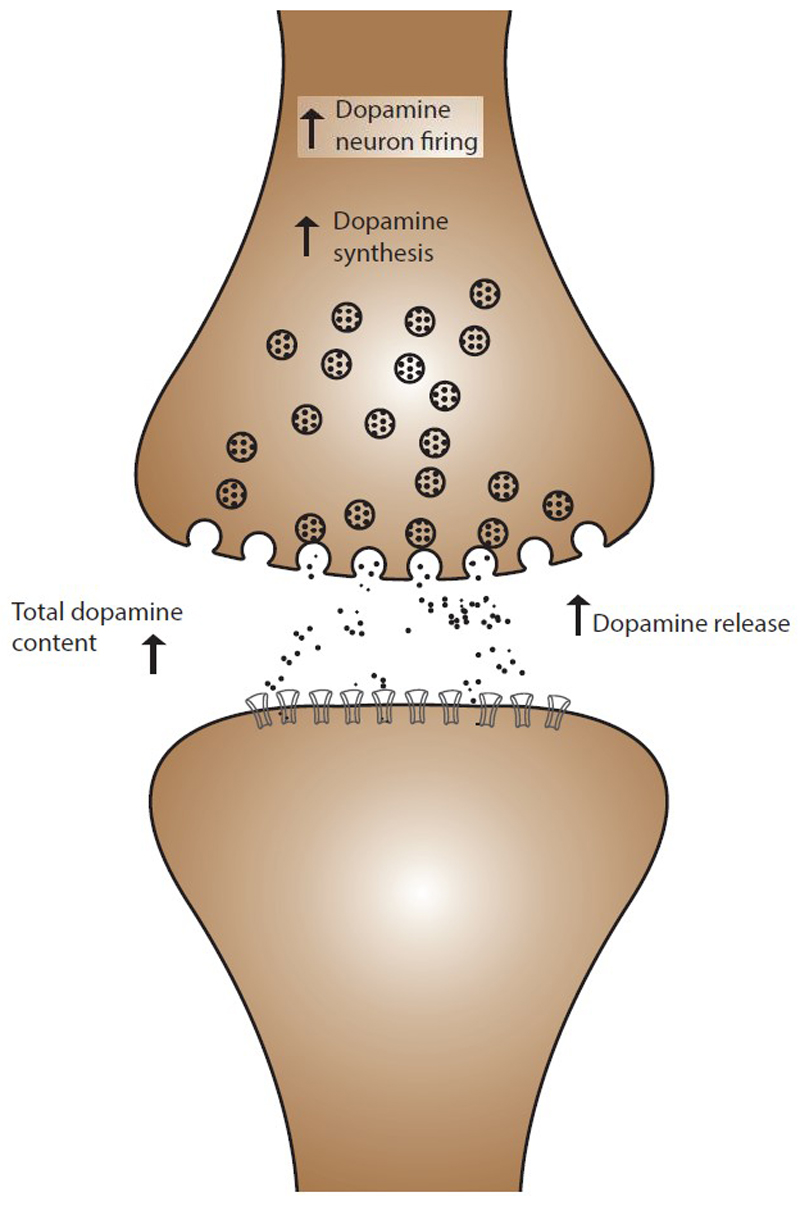
Fig. 2. Summary of the acute effects of THC on dopaminergic function.
In animal models acute THC challenge is associated with increased dopaminergic cell firing, increased dopamine synthesis and increased dopamine release.
Subsequent work investigated THC-induced increases in dopamine synthesis in vivo. THC increased [3H]-dopamine synthesis35,36, tyrosine hydroxylase37 and messenger ribonucleic acid (mRNA) expression38, the rate-limiting step in the dopamine synthesis pathway. Similarly, increases in dopamine metabolism, measured with the dihydroxphenylacetic acid/dopamine (DOPAC/DA) ratio, were reported in most39 but not all37 rodent studies. However, the majority of early studies using spectrophotofluorimetry were inconsistent due to technical limitations in detecting the rapid changes in extracellular dopamine concentration detectable by microdialysis techniques used more recently40.
In vivo microdialysis shows that acute THC increases dopamine efflux in the prefrontal cortex (PFC)41, striatum42 and nucleus accumbens (NAc)43. Only one study did not find THC–induced increases in dopamine efflux44, which may have been related to route of administration since that study used a THC gavage whereas the other studies used intravenous injection which produces a rapid increase in THC which reaches the brain promptly compared to gavage which favours sequestration in lipid compartments due to the very high lipid solubility of THC45. Differences in microdialysis results are associated with the strain of experimental rat46. Electrophysiological studies in rats have categorically demonstrated that THC dose-dependently increases firing rates in ascending midbrain dopaminergic projections via CB1R agonism47,48. Taken together, these findings suggest THC increases the firing rates of dopamine neurons which leads to increased dopamine synthesis and release in terminal fields.
Acute THC and post-synaptic dopamine in animals
Acute THC did not alter dopamine receptor proteins levels in rhesus monkeys49. In the rat limbic forebrain, one study reported increased dopamine type 1 receptor (D1R) availability50 whilst other studies reported decreases51. In the striatum, dopamine type 2 receptor (D2R) density showed either a decrease51 or no significant changes, whilst decreases in D1R have been reported50. Taken together, the findings of no change in receptor protein levels together with a tendency for reduced receptor availability most likely reflects THC-induced changes in synaptic dopamine levels. .
Acute THC and dopamine in humans
Studies of metabolic brain activity in humans using functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) provide indirect measures of dopaminergic function through changes in cerebral blood flow and glucose metabolism. These serve as surrogate markers of brain activity in areas with dopaminergic projections. In humans, acute THC is associated with increased activity in frontal and sub-cortical regions52. However, as the CB1R has the highest concentrations in these regions53 these findings may be due to direct endocannabinoid effects rather than reflecting dopamine-mediated processes. When studies of acute THC on resting brain activity have focussed on regions with dense dopaminergic innervation, such as the striatum, there have been inconsistent effects, with reports of both increased and reduced activity52. However, certain cognitive tasks are modulated by dopaminergic signalling and may provide a more robust proxy for THC-induced changes in dopaminergic transmission. For example, motor response inhibition is associated with cortical dopamine release and an fMRI study in healthy humans with previous exposure to cannabis found that THC attenuates activation in the right inferior frontal cortex and the anterior cingulate cortex (ACC) during suppression of motor inhibition54. Further indirect evidence of blunted dopaminergic processing comes from a study in healthy humans with previous exposure to cannabis using a verbal working memory task whereby THC attenuated striatal activation55 and a study of reward function in occasional cannabis users, which found that THC induced a widespread attenuation of the brain response to feedback in reward trials56 but not under reward anticipation conditions56.
It is also possible to directly measure the dopamine system using molecular imaging. These studies have examined the effect of acute THC on dopamine in humans with previous exposure to cannabis in vivo. Using PET, a combined analysis57 of two previous studies has shown that THC does indeed cause dopamine release in the ventral striatum in the human brain57. Likewise, acute THC challenge elicited dopamine release in fronto-temporal cortical brain regions58, although this finding requires replication with radiotracers that show higher affinity for cortical dopamine receptors. However, in a separate study using single photon emission computed tomography (SPECT) no significant THC-induced dopamine release was observed,59 which may be due to a relatively small sample size (n=9). This is because THC-induced dopamine release in the striatum appears to be of a lower magnitude than that caused by other drugs such as amphetamine and methylphenidate60, combined with difficulties in imaging dopamine changes that are comparatively small61.
Repeated THC and pre-synaptic dopamine
Studies of repeated THC dosing have yielded complex, regionally-specific effects including increased total striatal dopamine levels62 in rats but reduced hippocampal63 dopamine levels in mice. Reduced dopamine metabolism was found in the medial PFC in two rat studies62, an effect which was not observed in the striatum or the NAc64. THC administration for 21 days down-regulated tyrosine hydroxylase mRNA expression in the substantia nigra and ventral tegmental area (VTA) midbrain nuclei, but not in the cortex or striatum65. In the NAc, several studies have reported that multiple THC doses do not significantly change dopamine release66 in rats. However, this may be due to differential dopaminergic responses within the NAc, as in one rat study repeated THC doses led to increased dopamine release in the NAc core but decreased release in the NAc shell67. The picture is further complicated by genetic strain effects whereby increased dopamine release in the NAc was observed in Lewis but not Fischer 344 rat strains68. Taken together, these studies indicate that repeated THC dosing produces regionally-specific effects on dopamine function.
Human studies in cannabis users
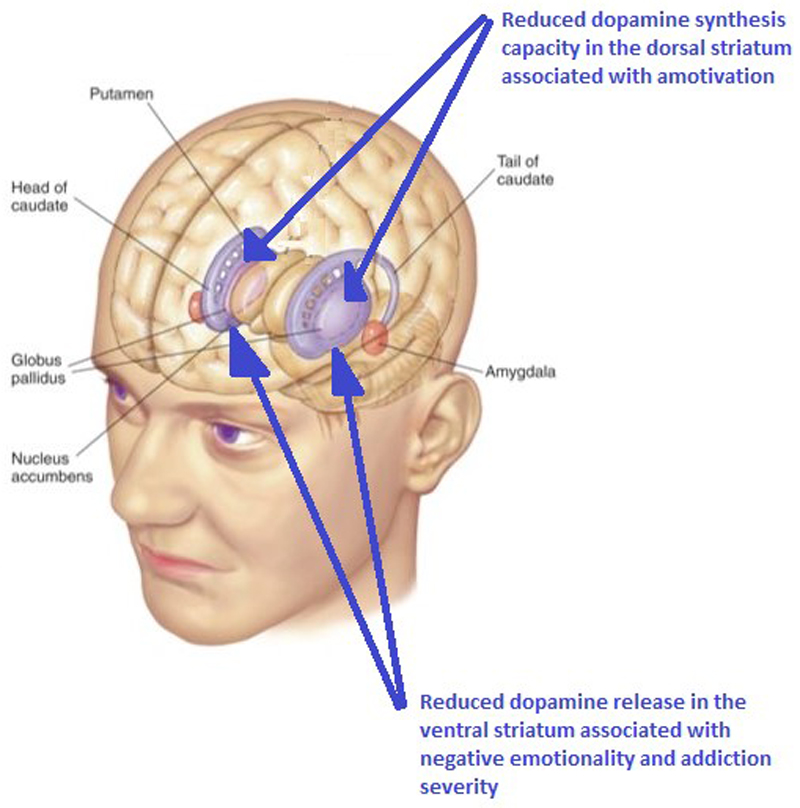
Several molecular imaging studies of dopaminergic function have been conducted in human cannabis users. Using PET, dopamine synthesis capacity was reduced in cannabis users69. Importantly, this reduction was driven by users meeting clinical criteria for abuse or dependence and was related to the severity of cannabis use (Fig. 3). Likewise, in two separate studies, cannabis users displayed reduced dopamine release to a stimulant challenge which was inversely related to severity of cannabis use70 and cognitive deficits including poor working memory71. Since no alteration in amphetamine-induced dopamine release was seen in recently abstinent cannabis users72, this effect is likely related to active use of the drug. While chronic use was not associated with altered stress-induced dopamine release73, there was evidence of a positive relationship between duration of cannabis use and stress-induced dopamine release in the limbic striatum73. Likewise, there is recent data showing that cannabis users have an attenuated metabolic response to methylphenidate challenge in the striatum, with a negative relationship between methylphenidate-induced metabolic increases and severity of cannabis use74. There is also evidence of reduced dopamine transporter (DAT) density in chronic cannabis users75. Whilst the interpretation of some of these studies is complicated by cannabis users also smoking tobacco, a recent experiment has addressed this by studying cannabis users without comorbid substance dependence, to show that cannabis users do indeed have reduced dopamine release71. Overall there is converging evidence for reduced presynaptic dopaminergic function in cannabis users.
Fig. 3. Cannabis use in humans is associated with reduced dopamine in the striatum. PET studies have shown lower striatal dopamine synthesis and release capacity in cannabis users.
Lower dopamine synthesis capacity in the dorsal striatum is directly associated with reduced motivational levels76 and reduced dopamine release in the ventral striatum is directly associated with negative emotion levels and addiction severity70
Repeated THC and post-synaptic dopamine
Studies in rats have reported that multiple THC dosing results in increased D2R availability in the midbrain, striatum and PFC65,77 and that this is associated with dopamine receptor sensitisation. There is further evidence of downstream dopaminergic effects of THC, as one study in rats78 reported up-regulated postsynaptic dopamine receptor signalling in the NAc via increased adenylyl cyclase activity, which was proposed to underlie THC-induced changes in amphetamine-induced locomotive behaviour. These findings suggest that repeated THC dosing results in altered dopamine receptor signal transduction.
Central Bank Digital Currency
CBDCs have the potential to radically transform the financial system, and all signs point to that transformation being a detriment to the individual users. A CBDC poses substantial risks to financial privacy, financial freedom, free markets, and cybersecurity. Yet the purported benefits fail to stand up to scrutiny. There is no reason for the government to issue a CBDC when the costs are so high and the benefits are so low. A completely developed Social Credit System is ready to be implemented in world through advanced surveillance.
Ask Aafreen Ghorbani a question now